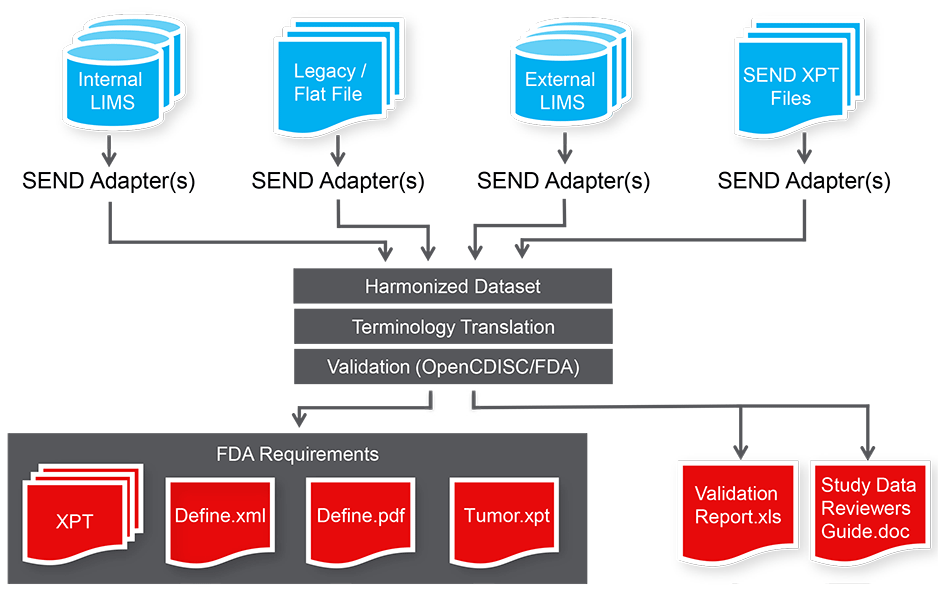
TranSEND™ is the all-encompassing software solution to get your preclinical data SEND-ready. TranSEND effectively aggregates and translates data fr
om multiple organizations and LIMS to produce one set of harmonized and validated SEND files, including define.xml and define.pdf, as required by the FDA.
In early 2014, PDS announced the release of the industry’s first FDA-validated, preclinical research SEND dataset made available to the public free of charge and qualification. The dataset can be used by scientists and research organizations across the industry to aid in validation and to serve as an example of a complete and FDA-compliant SEND dataset. The release of this anonymized dataset demonstrates our commitment to advancing electronic standards use and development in the noncompetitive space. The dataset is now available on the Pharmaceutical Users Software Exchange (PhUSE) website for use by the community.
Like all PDS solutions, TranSEND was built by a team of expert programmers and scientists. TranSEND is more than a necessary piece of software ? it’s a flexible and easy-to-use tool that simplifies the SEND translation process.
For example, TranSEND allows the use of multiple glossaries, each of which is mapped to required controlled terminology. Once initial mapping is done, versioning is simple as new controlled terminologies become available because of TranSEND’s unique self-learning technology. And when you choose TranSEND, we make sure it works to accelerate preparation of your submission package. Our team of toxicologists and IT personnel will partner with you to provide guidance that will smooth your transition to being SEND-ready.